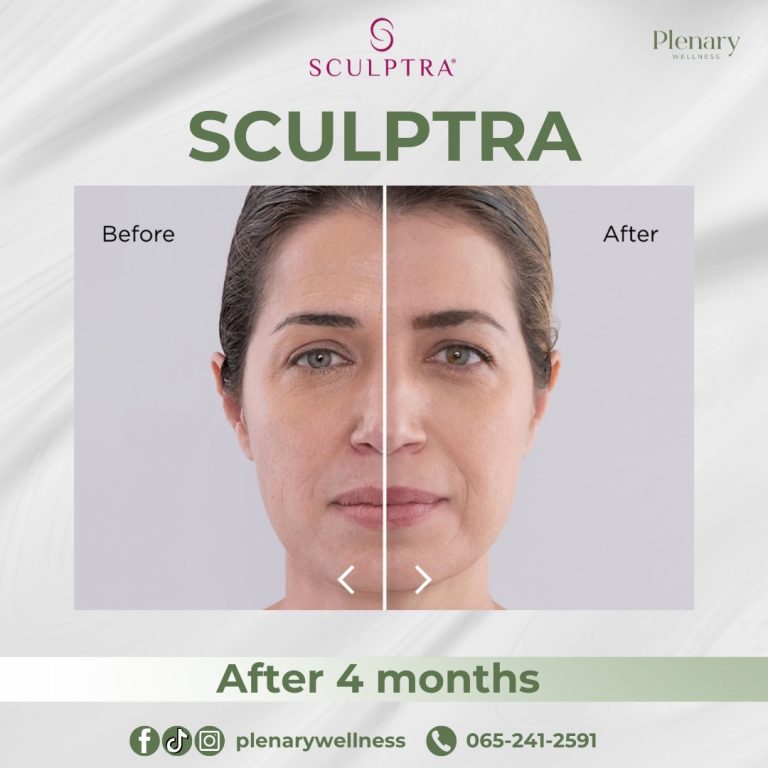
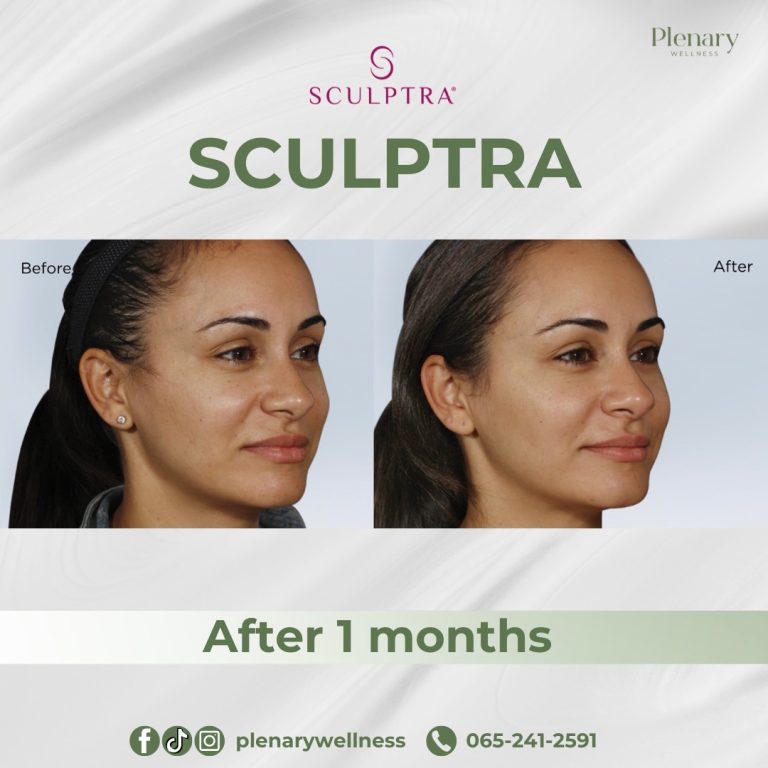
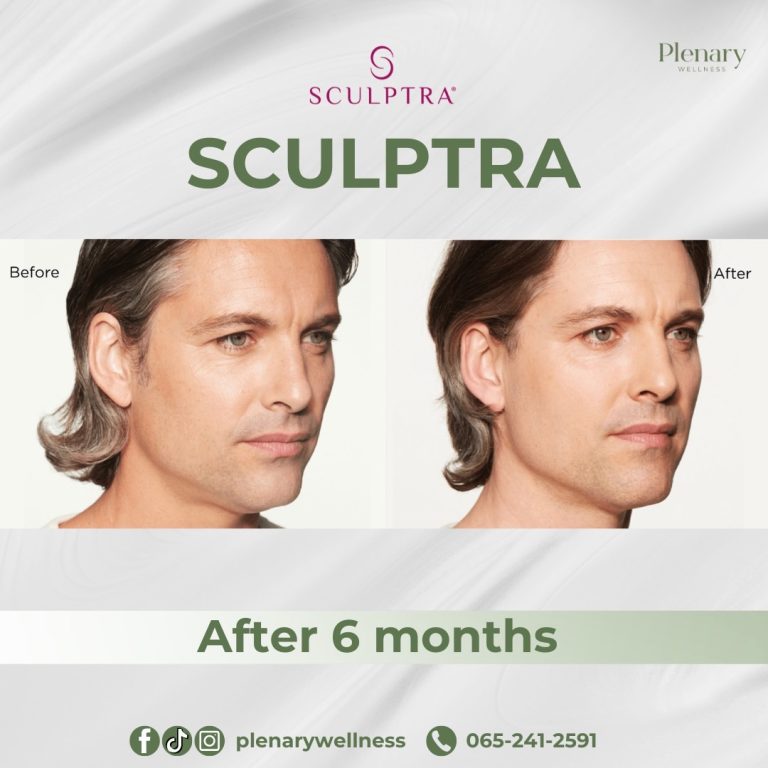
Sculptra is an injectable dermal filler that has been available since 1999. It was first FDA-approved in 2004 to treat Lipoatrophy in individuals living with HIV, a condition causing facial fat loss leading to sunken cheeks and deep folds and indentations on the face.
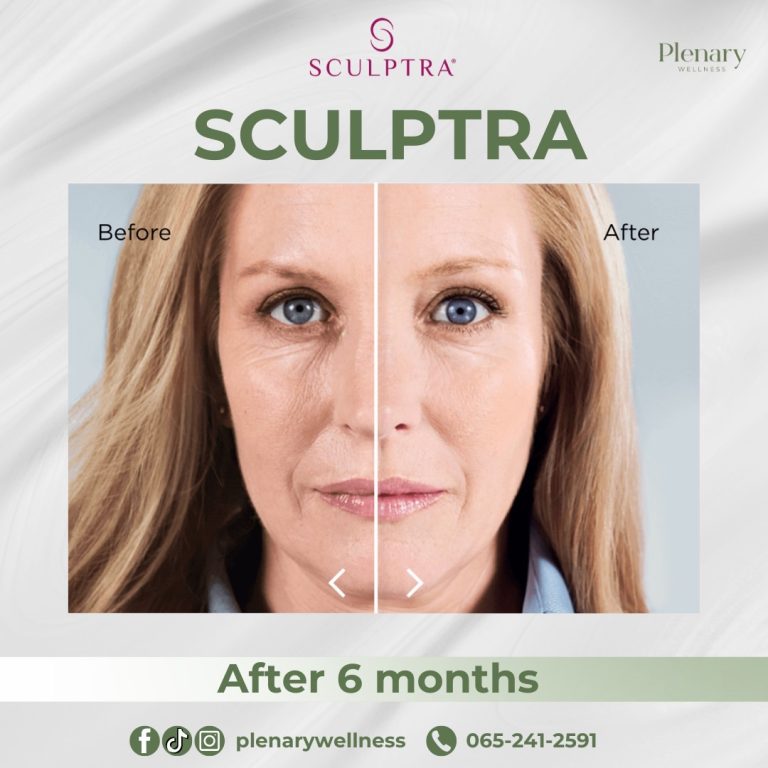
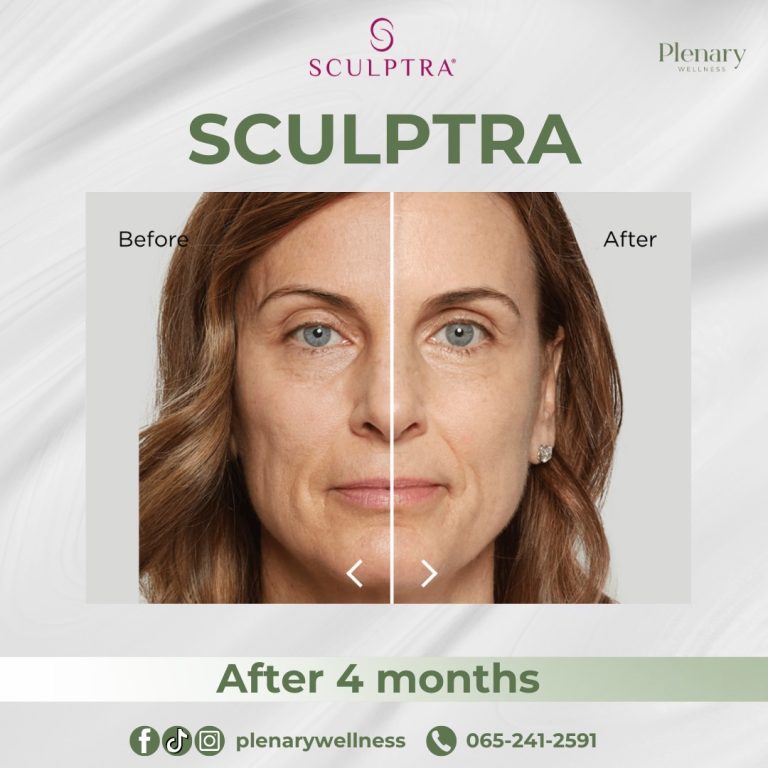
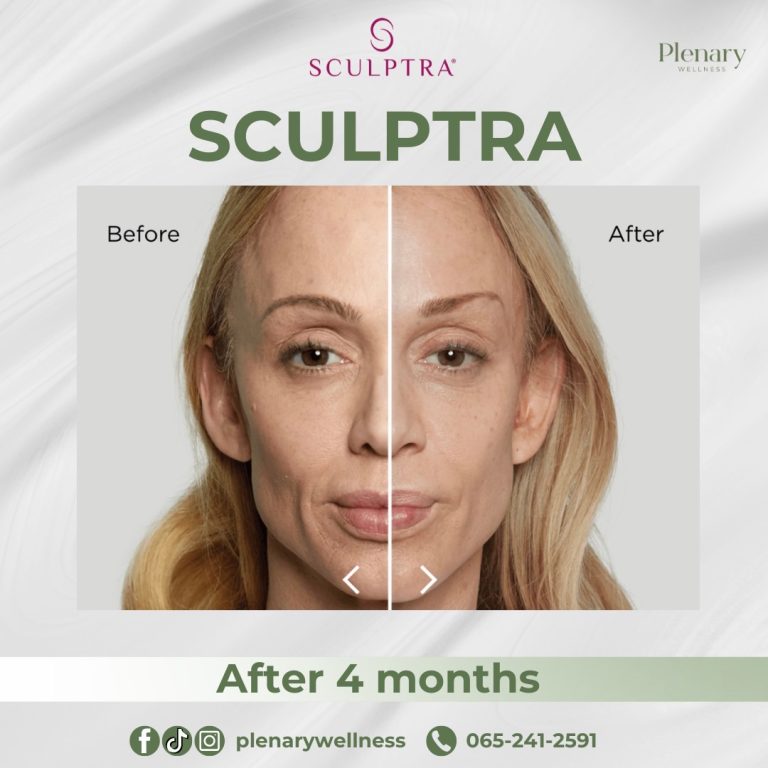
In 2014, the FDA approved Sculptra Aesthetic for treating wrinkles and folds on the face to provide a more youthful appearance.
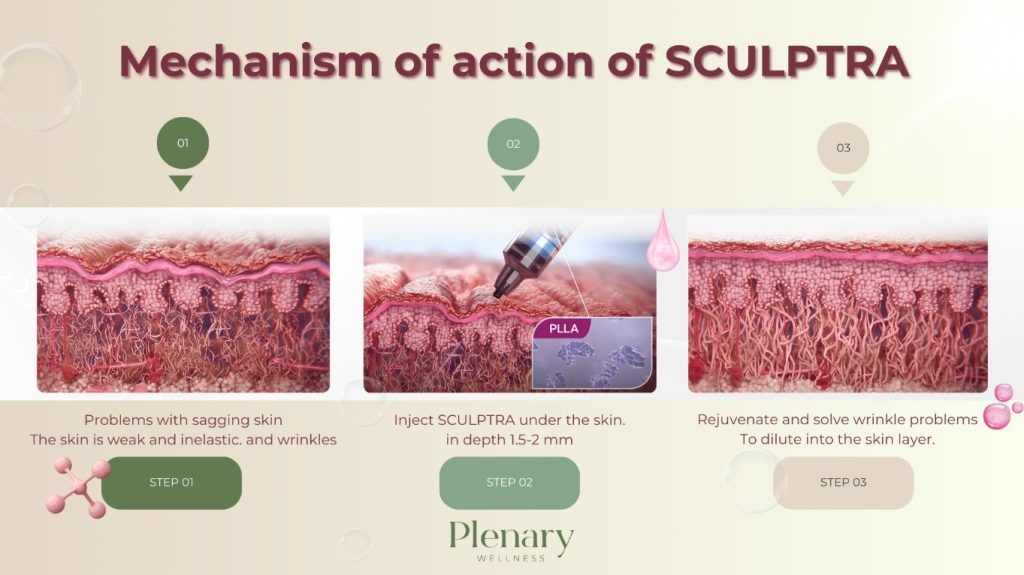
The main ingredient in Sculptra is poly-L-lactic acid (PLLA), classified as a collagen stimulator that delivers long-lasting, natural-looking results lasting up to two years. Sculptra is considered safe and effective, although it is not recommended for individuals with allergies to any of its ingredients or those with medical conditions causing irregular scarring.